Research news, Dec. 21st, 2012.
Cyclopentadiene-Phosphine/Palladium Catalyzed Cleavage of C-N Bonds in Secondary Amines: Synthesis of Pyrrole and Indole Derivatives from Secondary Amines and Alkenyl or Aryldibromides
Weizhi Geng, Wen-Xiong Zhang, Wei Hao, Zhenfeng Xi*
J. Am. Chem. Soc. 2012, 134, 20230-20233.
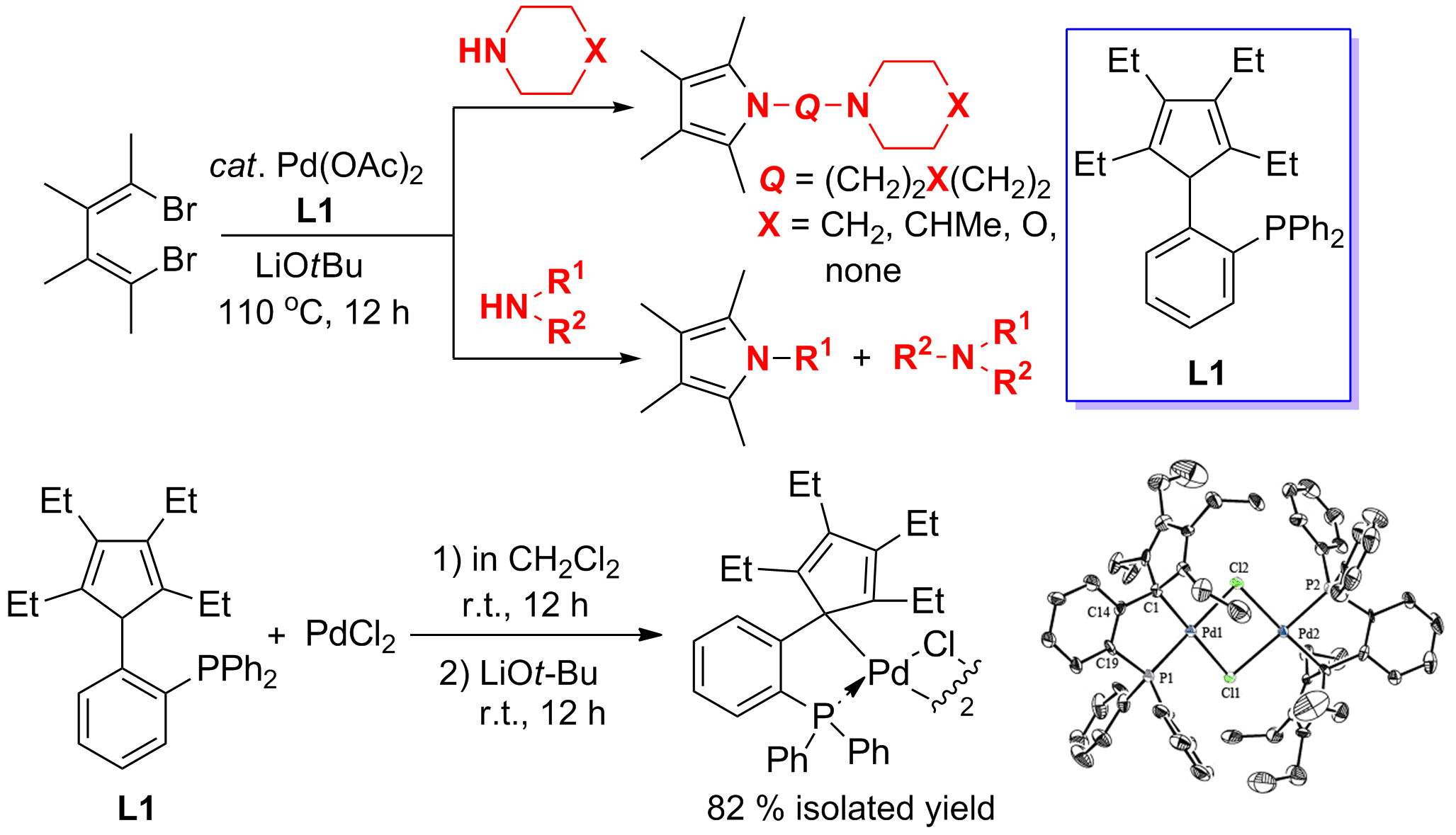
The cleavage of carbon–nitrogen (C–N) bonds is of significant synthetic interest, because such bonds are common in organic chemistry and usually unreactive. There was no report in the literature on transition-metal catalyzed cleavage and synthetic applications of C(sp3)–N bonds in secondary amines. Since a secondary amine contains one N–H bond and two C–N bonds, their efficient selective cleavage and further synthetic applications are very attractive for both mechanistic study and synthetic chemistry.
In this work, an efficient Pd–catalyzed cleavage of C(sp3)–N bonds in secondary amines and consequent C(sp2)–N and C(sp3)–N coupling process was developed. Various secondary amines could be used to react with alkenyl or aryl dibromides affording pyrroles and indoles in high yields. Cyclopentadiene-phosphine ligands, a new type of P–olefin ligands, were designed and found to be able to remarkably promote the efficiency of this Pd–catalyzed process. A reactive Pd complex coordinated with a cyclopentadiene-phosphine ligand was successfully isolated and structurally characterized.
亮点介绍
通过我们自己发展的方法,设计、合成独特的配体是本组近年来一直在思考的课题。本工作利用本组发展的方法(Xi, Z.; Li, P. Angew. Chem. Int. Ed. 2000, 39, 2950; Zhao, C.; Li, P.; Cao, X.; Xi, Z. Chem. Eur. J. 2002, 8, 4292.),设计合成了一类分子内含有多取代环戊二烯(双烯)以及膦原子的新型配体。利用该配体与钯的催化体系,首次实现了二级胺中Csp3-N键的催化选择性切断,合成了一系列N上具有不同取代基的吡咯和吲哚衍生物。利用同样的方法,我们合成的具有不同取代基的多取代环戊二烯也表现出不同与一般环戊二烯配体的独特性质(Xu, L.; Wang, Z.; Zhang, W.-X.; Xi, Z. Inorg. Chem. 2012, 51, 11941)。
膦-烯双配配体近年来在过渡金属催化的有机合成反应中显示出独特性质。预期该类新型配体(膦-环戊二烯)将在更多的过渡金属催化的有机合成反应中显示独特的作用。
