Research, news, Apr. 23rd, 2013.
1,2,3,4-Tetrasubstituted Cyclopentadienes and Their Applications for Metallocenes: Efficient Synthesis via Zirconocene and CuCl Mediated Intermolecular Coupling of Two Alkynes and One Diiodomethane
Weizhi Geng, Chao Wang, Jie Guang, Wei Hao, Wen-Xiong Zhang, Zhenfeng Xi*
Chem. Eur. J. 2013, DOI: 10.1002/chem.201300416
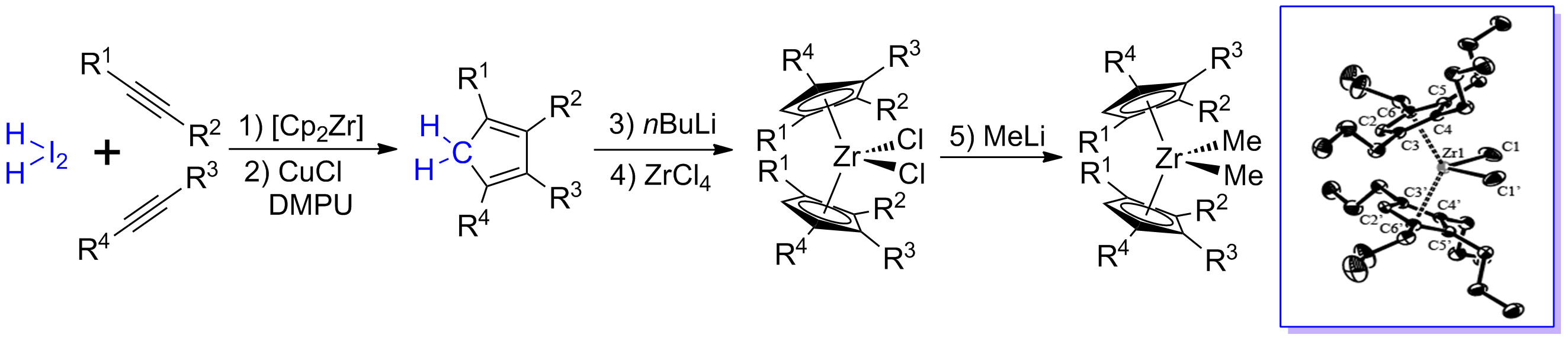
1,2,3,4-Tetrasubstituted cyclopentadienes and indene derivatives with identical or different substituents, were obtained in good to excellent isolated yields via a zirconocene and CuCl mediated intermolecular coupling process. This synthetic procedure involved three organic partners including one CH2I2, and two different or identical alkynes. Two alkynes or one diyne undergo Cp2Zr(II)-mediated pair-selective reductive coupling to afford their corresponding zirconacyclopentadiene derivatives, which react, in the presence of CuCl and DMPU, with CH2I2 via intermolecular followed by intramolecular coupling to afford the cyclopentadiene derivatives. Application of thus prepared tetra-substituted cyclopentadiene derivatives was demonstrated by the facile synthesis of their corresponding zirconocene complexes (4RCp)2ZrCl2, (4RCp)2ZrMe2, (4RCp)2ZrEt2 and (4RCp)2ZrBu2. The unique 1,2,3,4-tetrasubstituted cyclopentadiene ligands and their corresponding metallocenes are expected to have further applications in organometallic chemistry and organic synthesis.
