Research news, Feb. 10th, 2015
Synthesis of α,α,α’,α’-Tetrachloro Δ1-bipyrrolines and 4,8-Dichloro 2,6-diazasemibuvallenes
Ming Zhan, Shaoguang Zhang, Zhe Huang, and Zhenfeng Xi*
Org. Lett. 2015, DOI: 10.1021/acs.orglett.5b00136 (online 20150209)
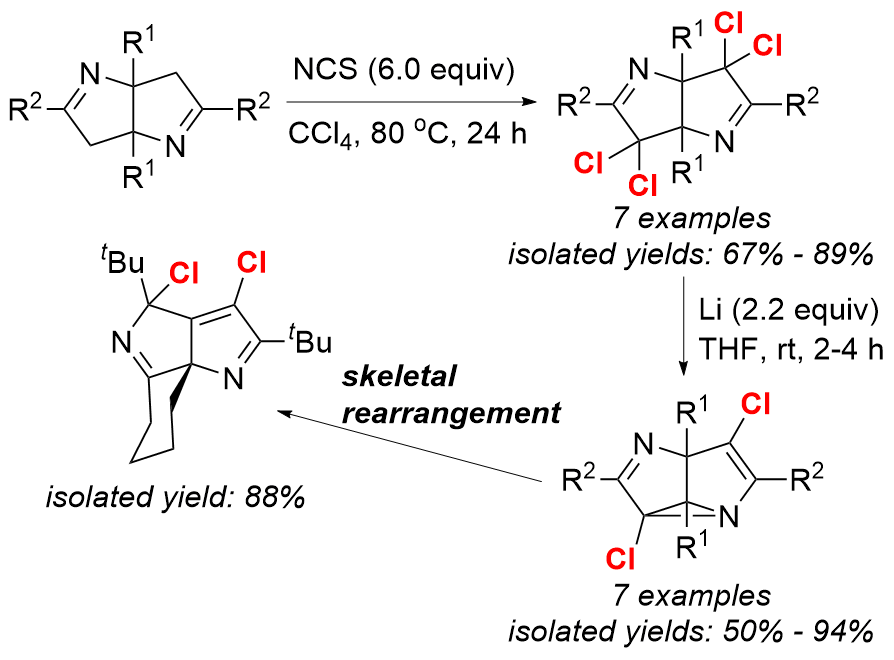
2,6-Diazasemibullvalenes (NSBVs) are interesting both theoretically and experimentally, because such highly strained ring systems demonstrate extremely rapid aza-Cope rearrangement and have been predicted to possess homoaromatic delocalized structures. However, due to their inherent structural instability, the studies on NSBVs including their synthesis, reaction chemistry, and synthetic application are very limited. In 2012, we established first synthetic method for NSBVs and studied their structures and reaction chemistry. However, more diversified NSBV derivatives with different substituents are needed to investigate their physical and chemical properties. Halogenated NSBVs attracted our attention, because the electron-withdrawing halide substituents might have unprecedented effect on both the rate of aza-Cope rearrangement and their further reaction chemistry, which would make such compounds different from the corresponding non-halogenated ones.
A series of 4,8-dichloro 2,6-diazasemibullvalenes were synthesized and isolated from the reaction of α,α,α’,α’-tetrachloro Δ1-bipyrrolines with lithium via C−N bond formation. All those dichloro diazasemibullvalene derivatives demonstrated extremely rapid aza-Cope rearrangement in solution. An unprecedented skeletal rearrangement of 4,8-dichloro 2,6-diazasemibullvalene derivatives took place, resulting in the formation of a new bipyrroline skeleton.
