Research news, Mar. 5th, 2014.
Palladium-Catalyzed One-Pot Three or Four-Component Coupling of Aryl iodides, Alkynes and Amines via C-N Bond Cleavage: Efficient Synthesis of Indole Derivatives
Wei Hao, Weizhi Geng, Wen-Xiong Zhang, and Zhenfeng Xi*
Chem. Eur. J. 2014, 20, 2605-2612.
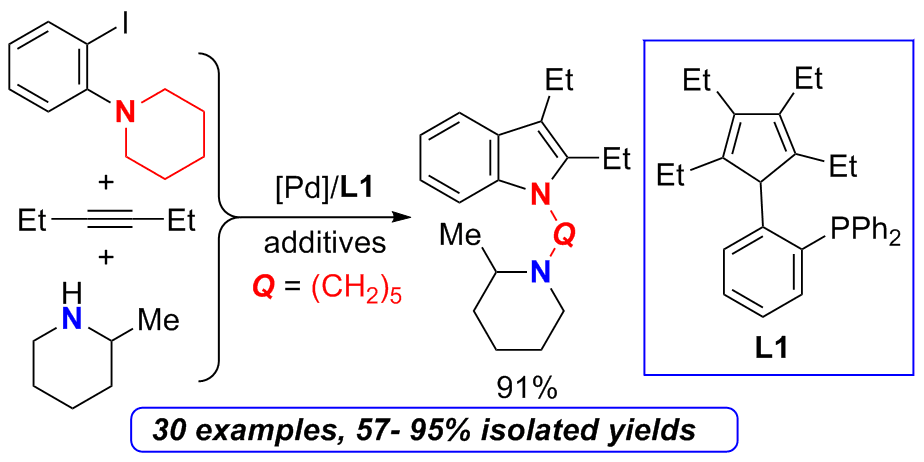
An efficient synthesis of N-substituted indole derivatives was realized by combining the Pd-catalyzed one-pot multi-component coupling approach with cleavage of the C(sp3)-N bonds. Three or four components of aryl iodides, alkynes and amines were involved in this coupling process. The cyclopentadiene-phosphine ligand showed high efficiency. A variety of aryl iodides, including cyclic and acyclic tertiary amino aryl iodides, and substituted 1-bromo-2-iodobenzene derivatives could be used. Both symmetric and unsymmetric alkynes substituted with alkyl, aryl, or trimethylsilyl groups could be applied. Cyclic secondary amines such as piperidine, morpholine, 4-methylpiperidine, 1-methylpiperazine and 2-methylpiperidine, acyclic amines including secondary and primary amines all showed good reactivity. Further application of the resulted indole derivatives was demonstrated by the synthesis of benzosilolo[2,3-b]indole.
