Research news, Sep. 13th, 2013.
Alkaline-Earth Metallocenes Coordinated with Ester Pendants: Synthesis, Structural Characterization, and Application in Metathesis Reaction
Heng Li, Wen-Xiong Zhang, and Zhenfeng Xi*
Chem. Eur. J. 2013, 19, 12859-12866.
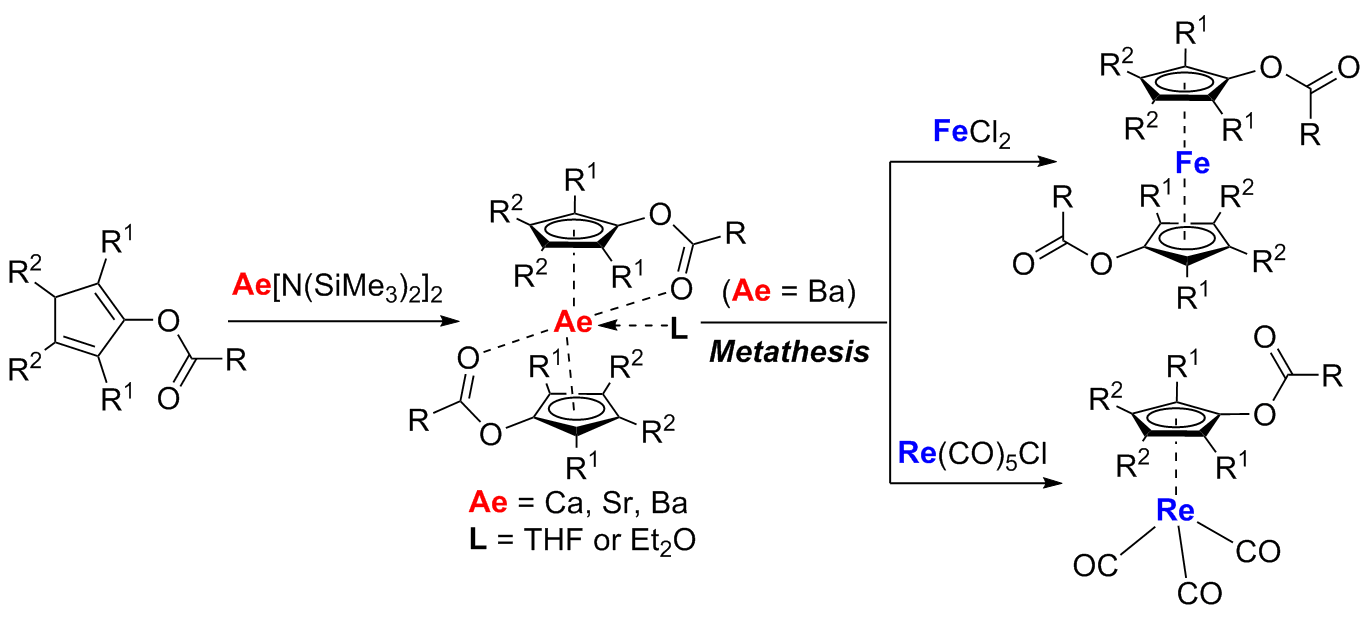
A variety of ester-substituted cyclopentadiene derivatives were synthesized in one-pot from the reaction of 1,4-dilithio-1,3-butadienes, CO and acid chlorides. Direct deprotonation of the ester-substituted cyclopentadienes with Ae[N(SiMe3)]22 (Ae = Ca, Sr, Ba) efficiently generated a new type of heavier alkaline-earth (Ca, Sr, Ba) metallocenes in good to excellent isolated yields. Single-crystal X-ray structural analysis demonstrated that these heavier alkaline-earth metallocenes were featured with two intramolecularly coordinated ester pendants and multiply substituted cyclopentadienyl ligands. Their corresponding transition metal metallocenes, such as ferrocene derivatives and half-sandwich cyclopentadienyl tricarbonylrhenium complexes, could be generated highly efficiently via metathesis reactions. The multiply substituted cyclopentadiene ligands containing an ester pendant, and their corresponding heavier alkaline-earth and transition-metal metallocenes would have further applications in coordination chemistry, organometallic chemistry and organic synthesis.
