Research news, Jul. 07th, 2013.
Palladium-Catalyzed Cleavage of the Me-Si Bond in ortho-Trimethylsilyl Aryltriflates: Synthesis of Benzosilole Derivatives from ortho-Trimethylsilyl Aryltriflates and Alkynes
Tianhao Meng, Kunbing Ouyang and Zhenfeng Xi*
RSC Adv. 2013, DOI: 10.1039/C3RA42910E
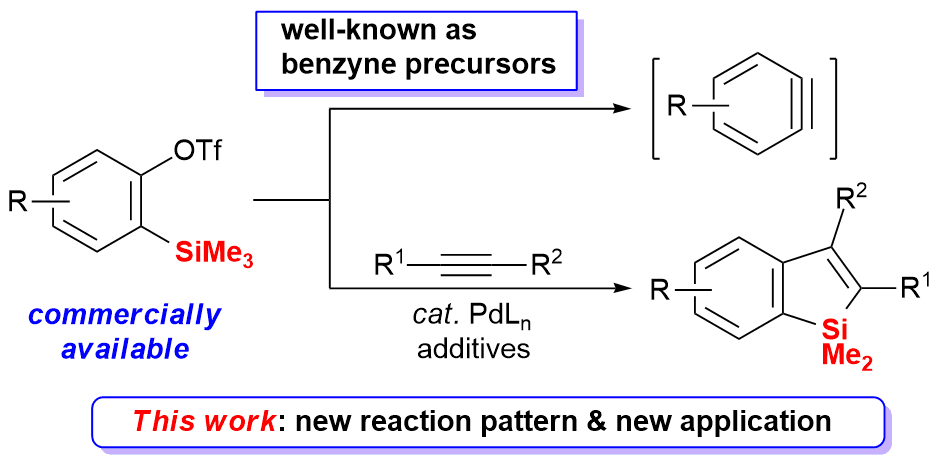
An efficient Pd-catalyzed cleavage of the Me-Si bond in ortho-trimethylsilyl aryltriflates was realized and synthetically applied. Most of commercially available ortho-trimethylsilyl aryltriflates could undergo the Pd-catalyzed intermolecular coupling with alkynes via cleavage of the Me-Si bond, which represents a new reaction pattern of ortho-trimethylsilyl aryltriflates. Potassium bromide (KBr) was found effective for this process. A variety of benzosilole derivatives were thus generated in high yields.
Transition-metal catalyzed selective cleavage of the Me-Si bond in a SiMe3 group and synthetic applications have attracted much recent interest. However, although remarkable achievements have been made, this research area is still at a very earlier stage, with much limitation on the diversity of suitable SiMe3-substituted substrates and reaction types. Further development of the transition-metal-catalyzed coupling accompanied by a selective cleavage of the C(sp3)-Si bond would lead to useful protocols for the synthesis of substituted siloles and derivatives, which are very important as organic materials in electronic and optoelectronic devices.
