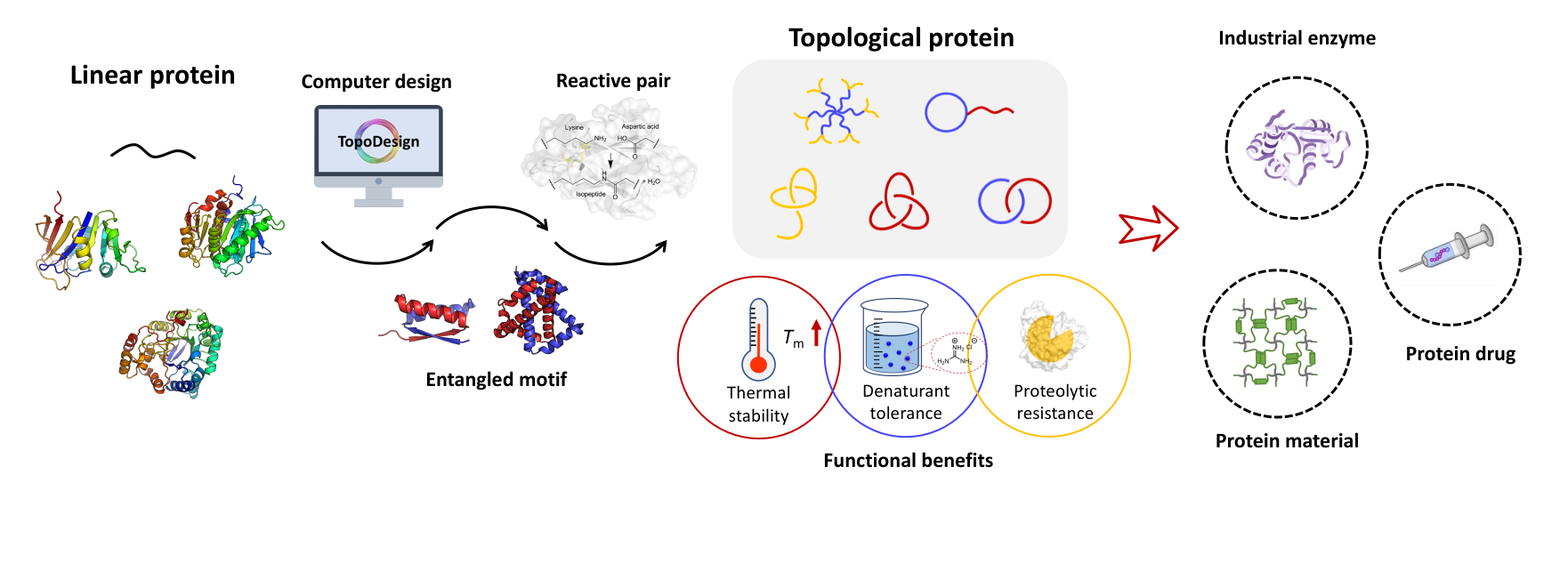
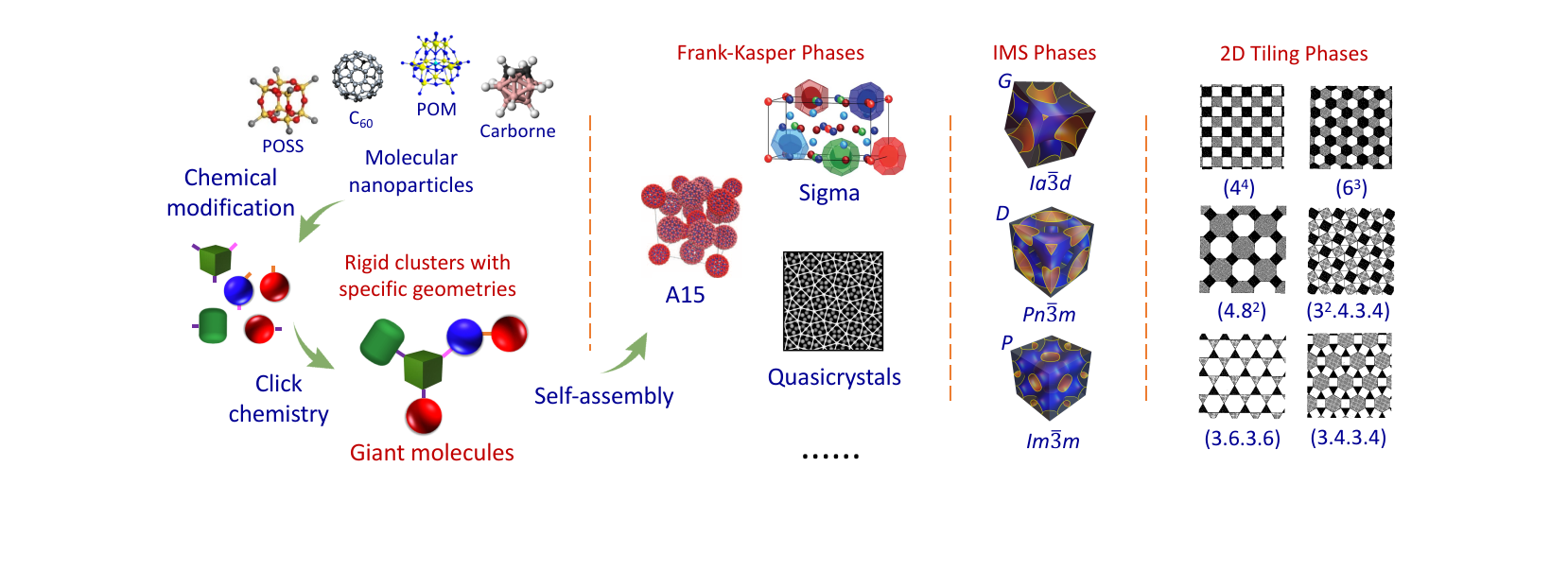
Quotes
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
Research Projects
Collaborations
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
Publications
Pei, J.;* Wang, J.-L.; Cao, X.-Y.; Zhou, X.-H.; Zhang, W.-B. Star-Shaped Polycyclic Aromatics Based on Oligothiophenes-Functionalized Truxene: Synthesis, Properties and Facile Emissive Wavelength Tuning, J. Am. Chem. Soc. 2003, 125, 9944-9945. [Link] [PDF]

Abstract
A facile approach to soluble star-shaped oligothiophene-functionalized polycyclic aromatics based on truxene is developed in this Communication. The Suzuki coupling reactions afford the thiophene-containing polycyclic aromatics with long branches (about 2.1 nm length from the heart to the periphery) from truxene precursor with excellent yields. The unsubstituted α-positions of thiophene rings allow for efficient halogenation and for further functionalization. The investigation of proton NMR spectra indicates that the hexahexyl groups efficiently prevent the self-association through the arene−arene π-stacking. Chemical shifts belonging to methylene groups move more upfield than do those of methyl groups. These chemical shift values (about 0.5−0.6 ppm) are quite lower than those of normal methyl and methylene groups. We also prepare a dendritic hyperbranched polymer P1 through FeCl3 mediated oxidative polymerizations. The photophysical properties of all compounds possessing good symmetry are investigated by UV−vis and emission measurement.